Order 222 PDF Template
Stop searching and find out why people love the ease of creating beautiful and legally compliant Order 222 PDF with PDFSimpli.
Stop searching and find out why people love the ease of creating beautiful and legally compliant Order 222 PDF with PDFSimpli.




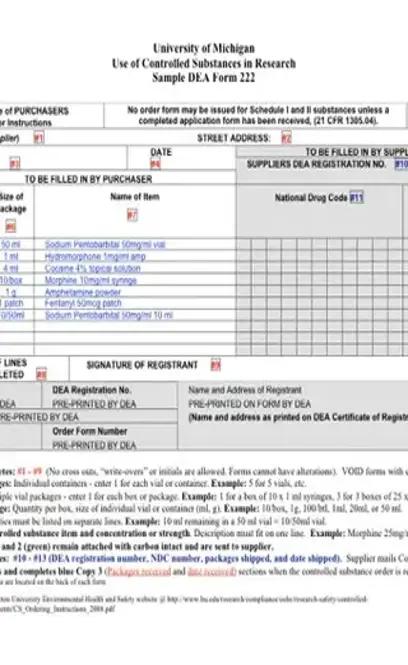
The DEA Order 222 Form (sometimes known as an Order 222 Form) is a part of the Drug Enforcement Agency’s Controlled Substances Ordering System. Those who wish to purchase Schedule I and II Controlled Substances such as doctors, pharmacies, or distributors must fill out and submit Order 222 Forms to the DEA. This order can be found in section 308 of the Act (21 U.S.C. 828).
The Order 222 Form is a requirement for any person, institution, or organization, whether private or otherwise, involved in the sales, distribution or manufacturing of any Schedule I and II Controlled Substances. By legal definition of the Controlled Substances Act (CSA) of 1970, a Schedule I substance is considered to be any drug, materials, or chemicals that currently have no accepted medical use. Furthermore, such substances have a high propensity for addiction and abuse. A few examples of Schedule I substances are listed below.
Methaqualone
Methylenedioxymethamphetamine (ecstasy)
Marijuana (cannabis)
Lysergic acid diethylamide (LSD)
Heroin
Peyote
As per Section 1305.04 of Title 21, Chapter II, Part 1305 Orders For Schedule I and II Controlled Substances, only persons who are registered with the DEA under section 303 of the Act (21 U.S.C. 823) can handle Schedule I and II Controlled Substances. Additionally, only individuals registered with the DEA under section 1008 of the Act (21 U.S.C. 958) and are with this authorized to export such substances may receive and use Order 222 Forms PDF or issue electronic orders for such substances.
[pdf-embedder url=”https://cdn-prod-pdfsimpli-wpcontent.azureedge.net/pdfseoforms/pdf-20180219t134432z-001/pdf/order-222-forms.pdf”]
Orders for Schedule I and II Substances may only be issued to the person whose name is registered with the DEA to receive those orders. Furthermore, this only applies if the person’s registration has not expired or been revoked. According to Section 1305.05 Power of Attorney, the registrant can authorize one or more people, whether or not they are located at the registered address, to purchase or issue orders of Schedule I and II Substances through power of attorney. A power of attorney must be retained in the files of the DEA along with any with executed Forms 222. Furthermore, the person should have on hand a power of attorney upon inspections.
Per Section 1305.13 Procedure For filling DEA Forms 222 the following steps should be done when filling out a DEA Form 222.
You must submit copy one and two of the DEA Form 222 to the supplier and retain copy three in the purchaser’s files.
If the supplier can fill the order and wishes to do so, they must record on copies one and two the number of items and the daye on which the items were shipped to the receiver. If for some reason the supplier cannot fill the order or has chosen not to, the form may be filled in part or an explanation given to as why the order was not filled. The supplier must fill the DEA Form 222 within 60 days of the date on the form, whatever the result is. No DEA Form 222 is valid past the 60 days.
The order can only be shipped to the person who issued the DEA Form 222 at the address on file with the DEA.
The supplier must keep copy one and send it to the agent in charge at the DEA’s field office nearest to the supplier.
The receiver must record on copy three how many items he or she received from the supplier along with the date received.
Persons who are entitled to use an Order 222 Form are those individuals who meet the following.
They are dispense the substances.
They are exporting the substances.
They are disposing of the substances.
They wish to return the substances to the supplier.
They are registered with DEA to dispense Schedule II Substances and wish to distribute said substances to another dispenser.
They are registered with the DEA to conduct chemical analysis or research involving controlled substances, and they want to distribute a Schedule I or II Controlled Substance to another individual who is also registered with the DEA to conduct chemical analysis, research, or provide instructional activities with said substances.
They are authorized by the DEA to handle Schedule II narcotics as a compounder.
They are authorized to electronic orders for distribution of narcotics to off-site treatment programs.
Being that Schedule I and II Controlled Substances are drugs under the regulation of the Drug Enforcement Agency, as well as state and local authorities, any unauthorized handling of such substances may result in legal action against someone who does not use the appropriate procedures. Pharmacies, doctors, laboratories, educational institutions, and any other individual or organization are required to use DEA 222 Forms to avoid sanctions, fines, and criminal charges. The use of such forms is also meant to prevent Schedule I and II Controlled Substances from getting into the wrong hands and sold on the black market.
Yes. As long as the registrant’s full named and address are understood and on record with the DEA, minor misspelled words should not affect its validity. In the case of a form being illegible, the distributor should request corrections where applicable.
The only things that can be filled in by the distributor are the date of the form, package size and type and strength of the substance.
Yes. As long as there is no question as to what was ordered versus what was sent.